NDC is conducting an ongoing study in conjunction with the U.S. Food and Drug Administration (FDA) to understand the effects of oxide layer composition and radial compression on corrosion resistance and biocompatibility of Nitinol medical devices. Nitinol is commonly used in medical devices including peripheral stents, stent grafts, and heart valves, due to its superelastic and shape memory properties. While previous studies have shown the significance of surface processing on oxide layer composition and its effect on localized corrosion behavior in Nitinol, the impact on nickel ion release and biocompatibility has yet to be studied. The first phase of the study focuses on filling this knowledge gap by investigating the relationship between post-manufacturing surface composition and the impact of post-manufacturing handling, to effectively simulate the impact of loading implantable devices onto a delivery system, on in-vitro corrosion behavior.
As part of the study, five groups of laser-cut Nitinol stents were produced by NDC using several processing methods to alter the oxide layer. The stents differed in surface condition and processing steps: oxidized tube shape set in salt pot (OT), ground tube shape set in air furnace (AF), ground tube shape set in salt pot (SP), ground tube shape set in salt pot followed by mechanical polish (MP), and ground tube shape set in salt pot followed by electropolish (EP). For each group, radially compressed and non-compressed devices were tested for nickel ion release over a 60-day immersion test. Additionally, pitting corrosion testing, oxide layer characterization, and visual inspection were performed on each group.
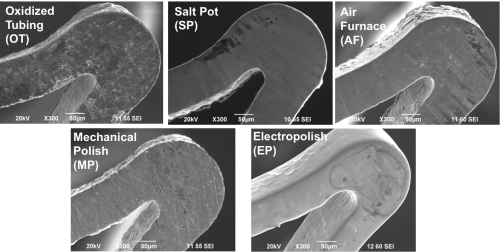
The results of the study indicate that additional nickel release from radial compression is dependent upon surface processing, and when compressed, thicker oxide layers are more prone to cracking which exposes nickel-rich regions and leads to greater ion release. The MP and EP groups, characterized by thin oxide layers, showed high corrosion resistance and were not negatively impacted by radial compression. The OT group, with minimal surface processing, resulted in the thickest oxide layer, low pitting, uniform corrosion resistance, and was negatively impacted by radial compression. These results demonstrate the significance of various surface treatment steps on a Nitinol device’s corrosion resistance and response to radial compression.
Christine Trepanier, Sr. Director Process Engineering noted, “This study is the first to establish the complex relationship between oxide layer composition and its impact on nickel ion release. We’re currently working on the next phase of the study, which investigates the biological effects of Nitinol’s oxide layer composition systemically and locally on arterial vessels in animals. The study is expected to conclude in mid-2016.”
Click here to download the full paper. For more information about NDC, please contact [email protected].