NDC has developed a new chemical etching process to preferentially remove thermal oxide from the surface of Nitinol based medical device implants. This breakthrough represents another technology NDC can utilize to tailor manufacturing processes specifically to customers’ unique and proprietary devices and is particularly suited towards smaller geometry devices, such as devices developed for neurovascular applications, and braided Nitinol devices.
One of the critical steps in Nitinol implant manufacturing involves exposing the Nitinol to high temperatures to achieve the desired final geometry, which creates a thermal oxide layer on the surface of the material. Traditional methods of thermal oxide removal include mechanical polishing and chemical etching. Although mechanical polishing presents a suitable option and is ideal for creating a uniform surface finish, the surface characteristics post-processing can be variable and inconsistent. In addition, mechanical polishing may not be suitable for smaller devices. Chemical etching is often favored for its ability to selectively attack and dissolve oxides which allows better process capability, in addition to its ability to treat smaller geometry devices and particularly those with tight intradoses. The drawback of standard chemical etching is that it can result in the dissolution of oxide inclusions within the material creating small voids within the cross-section of the material.
NDC’s next-generation chemical etching process has the ability to selectively remove the thermal oxide from the surface without dissolving these oxide inclusions. The result is an exceptionally smooth, uniform surface layer free of thermal oxide. Christine Trepanier, Sr. Director Process Engineering noted, “This new chemical etch solution was developed to achieve the best results that can be obtained from mechanical and chemical processes without any of the limitations.”
NDC’s innovative Nitinol etching process provides greater control, repeatability and consistency in manufacturing. Additionally, the process is well-suited for Nitinol devices with smaller geometries, small intradose radiuses and delicate structures. Devices utilizing braided Nitinol are also ideal candidates including: vascular stents, occlusion devices, basket filters, AAA and TAA components.
For more information on NDC’s next-generation chemical etching process, please contact [email protected].
Figures 1 and 2 below show component post-etch and post-EP respectively:
[row class=”nopad”]
[six-col class=”lnopad”]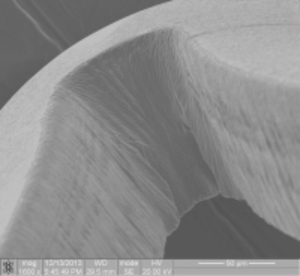 [/six-col][six-col class=”rnopad”]
[/six-col][six-col class=”rnopad”]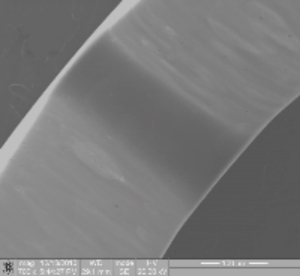 [/six-col]
[/six-col]
[/row]